Meta Title
3-TRENBOMED 150 (Trenbolone Blend) Clinical Reference Profile
Meta Description
Comprehensive 3-TRENBOMED 150 information for analytical and research purposes: pharmaceutical-grade trenbolone blend (150 mg/ml) intended strictly for laboratory reference, method development, and forensic toxicology. Not for human use.
Standalone Product Short Description
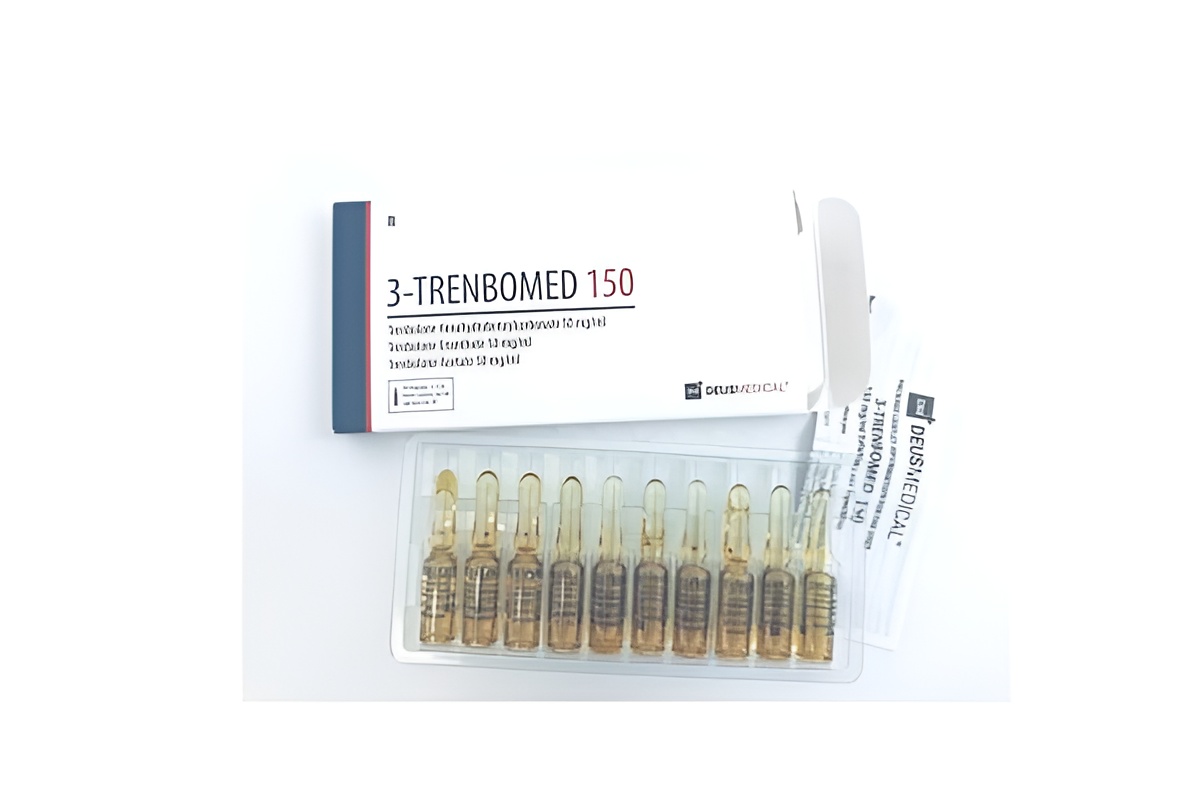
3-TRENBOMED 150 is a pharmaceutical-grade multi-ester trenbolone reference standard containing a precisely balanced blend of trenbolone acetate, trenbolone enanthate, and trenbolone hexahydrobenzylcarbonate at a total concentration of 150 mg/ml. Manufactured under strict GMP-like conditions, this Schedule III controlled substance is supplied exclusively as an analytical reference material for licensed laboratories, academic institutions, and regulatory agencies engaged in steroid profiling, doping-control, or pharmacokinetic research. All handling requires appropriate DEA/EU-equivalent licensing.
- Appearance: Clear, light-yellow to golden sterile oil solution
- Total Concentration: 150 mg/ml (50 mg/ml trenbolone acetate + 50 mg/ml trenbolone enanthate + 50 mg/ml trenbolone hexahydrobenzylcarbonate)
- Carrier Oil: Pharmaceutical-grade grapeseed oil with benzyl alcohol and benzyl benzoate
3-TRENBOMED 150 — Clinical & Pharmaceutical Reference Guide
Introduction
3-TRENBOMED 150 represents a sophisticated pharmaceutical-grade trenbolone blend designed specifically for advanced analytical and research applications. By combining three distinct trenbolone esters—acetate (rapid-acting), enanthate (intermediate), and hexahydrobenzylcarbonate (long-acting)—this reference material allows researchers to study complex multi-phase release kinetics, receptor-binding profiles, and metabolic pathways of 19-nor steroids in controlled laboratory settings.
Its exceptional purity (>99% total trenbolone esters via HPLC) and reproducible ester ratio make 3-TRENBOMED 150 a preferred standard in forensic toxicology laboratories, anti-doping agencies (WADA-accredited), and pharmaceutical development facilities investigating anabolic-androgenic steroid detection methods. This guide provides detailed 3-TRENBOMED 150 information strictly within the framework of legal, non-consumptive scientific use.
Key Features & Scientific Relevance
- Pharmaceutical-grade purity exceeding 99% (combined esters) verified by independent third-party laboratories
- Precise 1:1:1 ester ratio for consistent multi-phasic pharmacokinetic modeling
- Essential reference material for LC-MS/MS and GC-MS method validation in steroid screening
- Full compliance with DEA Schedule III, EU Annex I, and international controlled-substance regulations
- Supplied with batch-specific Certificate of Analysis (CoA) and full-scan NMR, FTIR, and mass spectra available upon institutional request
Product Specifications
- Chemical Names: Trenbolone acetate, Trenbolone enanthate, Trenbolone hexahydrobenzylcarbonate
- Molecular Formulas: C20H24O3 (acetate), C25H34O3 (enanthate), C26H34O4 (hexahydrobenzylcarbonate)
- Molecular Weights: 312.4 g/mol, 382.5 g/mol, 410.6 g/mol respectively
- CAS Numbers: 10161-34-9 (trenbolone base), 10161-33-8 (enanthate ester)
- Appearance: Transparent light-golden sterile oil
- Concentration: Exactly 150 mg/ml total trenbolone esters (50 + 50 + 50 mg/ml)
- Solubility: Fully miscible in ethyl oleate, ethanol, and other lipophilic solvents; insoluble in water
- Structural Classification: 19-nor testosterone derivative, Δ9,11 anabolic-androgenic steroid
Applications & Usage
In accordance with strict legal frameworks, 3-TRENBOMED 150 is employed exclusively in:
- Development and validation of multi-steroid analytical methods (LC-MS/MS, GC-MS)
- Forensic toxicology and workplace drug-testing panel calibration
- Pharmacokinetic studies of ester hydrolysis rates in vitro
- Anti-doping control reference material (WADA, IOC, NCAA)
- Research into trenbolone metabolism and urinary excretion markers
No therapeutic, veterinary, or performance-enhancement applications are permitted or implied.
Purity & Analytical Testing
Every batch of 3-TRENBOMED 150 undergoes exhaustive analytical verification:
- High-Performance Liquid Chromatography (HPLC-UV/DAD) – confirms exact 50/50/50 mg/ml ratio and >99% total purity
- Gas Chromatography-Mass Spectrometry (GC-MS) – full electron-impact fragmentation pattern matching
- Nuclear Magnetic Resonance (¹H-NMR & ¹³C-NMR) – complete structural confirmation of all three esters
- Fourier-Transform Infrared Spectroscopy (FTIR) – characteristic carbonyl and C=C stretch verification
These methods guarantee absence of synthetic byproducts, residual solvents, and heavy-metal contamination, ensuring maximum reliability for quantitative reference work.
Safety & Handling Guidelines
As a potent Schedule III controlled substance, 3-TRENBOMED 150 requires stringent laboratory safety protocols:
- Mandatory use of nitrile gloves, safety goggles, lab coat, and fume hood
- Storage at 2–8 °C protected from light; do not freeze
- Secondary containment and locked refrigeration mandatory
- Spill response: absorb with inert material, ventilate area, follow local hazardous waste disposal regulations
- Personnel must be trained in controlled-substance handling and maintain current DEA/research exemption documentation
Secure & Confidential Distribution for Institutions
Distribution occurs solely to verified institutional entities possessing valid controlled-substance licenses. Packaging features:
- Plain, unmarked exterior shipping containers
- Tamper-evident seals and temperature-monitoring devices
- No product identifiers on external labeling
- Interior documentation limited to CoA and chain-of-custody forms
All transactions comply with DEA Form 222 (U.S.) or equivalent international controlled-substance import/export permits.
Why Choose This Reference Material?
- Exact 1:1:1 ester ratio unmatched reproducibility in multi-phasic studies
- Full analytical data packet included (HPLC, GC-MS, NMR)
- Trusted by WADA-accredited and government forensic laboratories worldwide
- Immediate availability to qualified institutions with proper licensing
Legal & Safety Disclaimer
3-TRENBOMED 150 is supplied strictly as an analytical reference standard for licensed laboratories and academic institutions. It is not for human or veterinary use, not for sale to individuals, and not intended for consumption, injection, or any form of administration. Possession, transfer, or use without appropriate controlled-substance registration is illegal and may result in severe criminal penalties. All recipients must comply with the Controlled Substances Act (U.S.), Misuse of Drugs Act (U.K.), or equivalent national legislation.
FAQ Section
What is the exact composition of 3-TRENBOMED 150? 50 mg/ml trenbolone acetate + 50 mg/ml trenbolone enanthate + 50 mg/ml trenbolone hexahydrobenzylcarbonate in pharmaceutical-grade grapeseed oil.
Is 3-TRENBOMED 150 a controlled substance? Yes. Trenbolone and all its esters are DEA Schedule III (U.S.), Class C (U.K.), and Schedule IV (Canada) controlled substances.
How is the 50/50/50 ratio verified? Independent HPLC analysis with UV detection at 240 nm and 350 nm, confirmed by GC-MS.
What storage conditions are required? Refrigerate at 2–8 °C, protected from light. Stable for 36 months when stored correctly.
Who may legally acquire 3-TRENBOMED 150 reference material? Only institutions holding valid DEA research registration, EU controlled-drug license, or equivalent authorization from their national regulatory body.
Can this product be used for doping control calibration? Yes. It is routinely used as a calibrant and quality-control material in WADA- and NFL/NBA-accredited laboratories worldwide.




Reviews
There are no reviews yet.