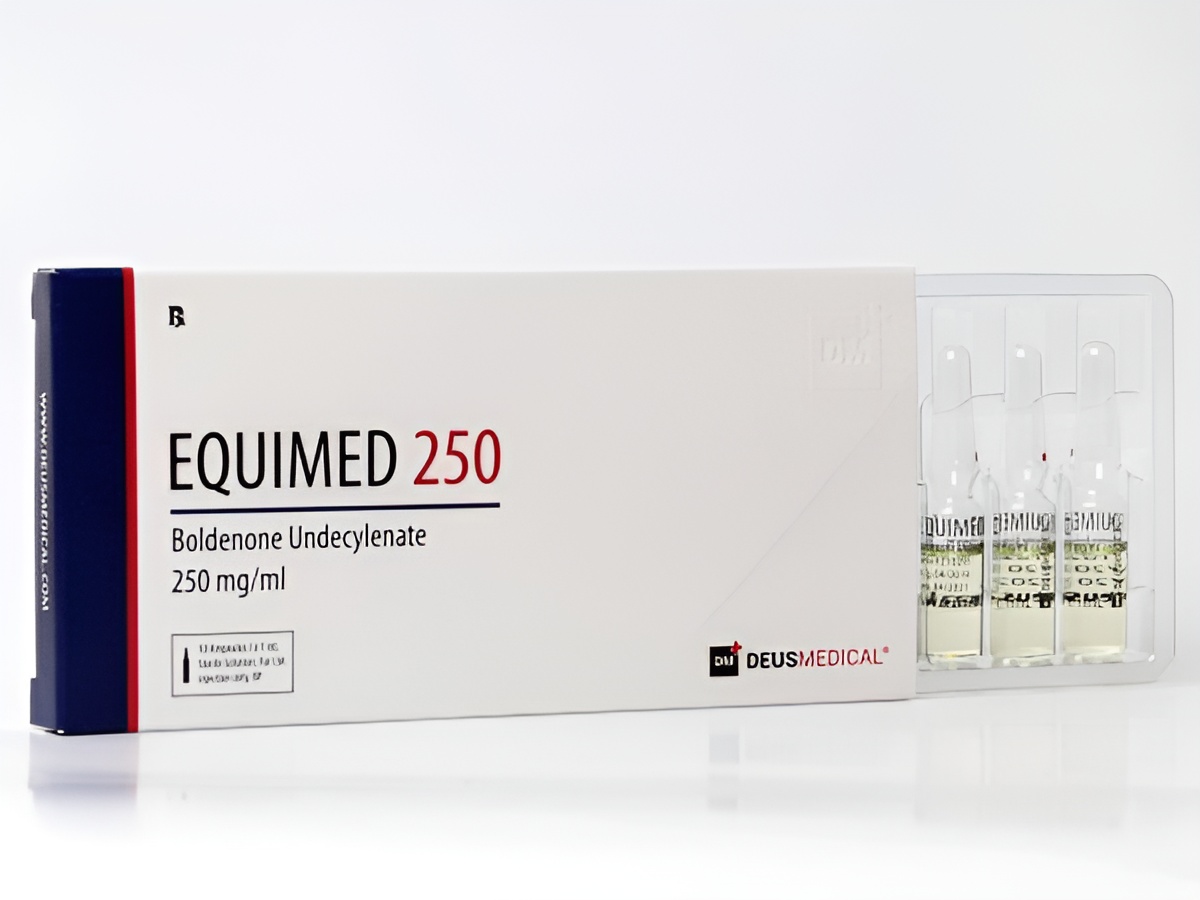
EQUIMED 250 by DEUS MEDICAL is a potent anabolic steroid designed for educational and research purposes, known for promoting muscle growth and enhancing performance.
Physical and Chemical Properties:
- Appearance: Clear, oily solution
- Molecular Weight: 344.49 g/mol
- Solubility: Soluble in oils; insoluble in water
- Chemical Formula: C22H32O3
- Structural Classification: Anabolic steroid
Introduction
EQUIMED 250 is a significant anabolic steroid produced by DEUS MEDICAL, widely utilized in laboratory research to investigate its effects on muscle hypertrophy, strength enhancement, and metabolic pathways. This formulation contains high pharmaceutical-grade purity, making it suitable for rigorous scientific studies.
Key Features & Scientific Relevance
Pharmaceutical-Grade Purity
EQUIMED 250 boasts pharmaceutical-grade purity exceeding 99%, essential for achieving accurate results in analytical applications. This level of purity ensures the integrity of laboratory trials.
Role in Laboratory Analysis
EQUIMED 250 serves as a critical tool in various scientific inquiries, allowing researchers to explore its effects on muscle development, hormonal balance, and metabolic responses. Its relevance in pharmacological studies underscores its value as a research resource.
Regulatory Compliance
EQUIMED 250 adheres to national and international standards, ensuring all substances used in research are compliant with legal requirements. Understanding these regulations is vital for everyone engaged in pharmaceutical research.
Secure and Confidential Handling Procedures
EQUIMED 250 is dispatched in secure, compliance-ready packaging to maintain confidentiality and security, accompanied by necessary documentation as legally required.
Product Specifications
- Appearance: Clear, oily solution
- Molecular Weight: 344.49 g/mol
- Solubility: Soluble in oils; insoluble in water
- Chemical Formula: C22H32O3
- Structural Classification: Anabolic steroid
Applications & Usage
EQUIMED 250 is utilized in various analytical contexts, significantly contributing to pharmacology and anabolic research. Claims regarding medical benefits should not be associated with its applications, as it is strictly intended for research purposes.
Purity & Analytical Testing
The purity of EQUIMED 250 is verified through advanced scientific methods:
- High-Performance Liquid Chromatography (HPLC): Assesses purity by effectively separating compound components.
- Gas Chromatography-Mass Spectrometry (GC-MS): Provides detailed compositional analysis for accurate identification.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Evaluates molecular structure and purity through interactions with magnetic fields.
- Fourier Transform Infrared Spectroscopy (FTIR): Analyzes molecular vibrations to confirm chemical identity.
These testing methods are crucial for ensuring reliability and consistency in research findings.
Safety & Handling Guidelines
As a controlled laboratory substance, EQUIMED 250 requires adherence to specific safety and handling protocols:
- Personal Protective Equipment (PPE): Laboratory coats, gloves, and safety goggles should be worn to minimize exposure.
- Storage Conditions: Store in a cool, dry place, away from direct light and moisture.
- Safety Data Considerations: Follow Material Safety Data Sheets (MSDS) for emergency response measures and risk management.
- Legal Handling Requirements: Handling must comply with all applicable laws regarding controlled substances.
Secure & Confidential Distribution
Licensed laboratories and academic institutions receive EQUIMED 250 through secure distribution channels. Packages include:
- Plain, Compliance-Ready Packaging: Protects contents and maintains confidentiality.
- Unmarked Exterior Labels: Ensuring discreet delivery.
- Documentation Included Only Where Legally Required: Providing essential information while maintaining compliance.
All handling and acquisition of EQUIMED 250 must comply with relevant national and local regulations.
Why Choose This Reference Material?
Opting for EQUIMED 250 provides researchers with:
- Accurate Scientific Data: Supported by rigorous methodologies and verified testing.
- Pharmaceutical-Grade Analytical Quality: Ensuring precise and reliable outcomes for studies.
- Trusted Reference Material for Academic or Clinical Professionals: Offering dependable resources for research and analysis.
- Compliant Documentation: Facilitating adherence to legal standards in research settings.
Legal & Safety Disclaimer
This compound is intended strictly for informational and professional reference only. It is not for human or veterinary use, nor is it available for sale or distribution. Handling requires appropriate licensing, with strict compliance with applicable regulations.
FAQ Section
What is EQUIMED 250, and what is it used for?
EQUIMED 250 is an anabolic steroid primarily used in scientific research to investigate its effects on muscle growth and performance enhancement.
How is EQUIMED 250 administered?
EQUIMED 250 is typically administered via injection, and specific dosage protocols depend on research objectives.
Are there common side effects associated with its use?
As a research compound, potential side effects typical of anabolic steroids may be considered, but the substance is not intended for personal use.
How should EQUIMED 250 be stored?
Store in a cool, dry environment away from direct light to maintain compound stability.
Who is authorized to handle EQUIMED 250?
Only licensed professionals and accredited institutions are permitted to handle and utilize EQUIMED 250 as a research material.
This comprehensive guide serves as a critical resource for professionals seeking detailed information about EQUIMED 250 by DEUS MEDICAL, ensuring adherence to legal standards in their respective fields.




Reviews
There are no reviews yet.